PADI GAS BLENDER COURSE
- Home
- PADI GAS BLENDER COURSE

PADI GAS BLENDER COURSE
More and more people are diving with enriched air and this means Padi Gas Blender course and the demand for enriched air fills is also increase. However, there needs to be someone qualified to administer these fills to meet this demand. This is where the DSAT Gas Blender course comes in. It can result in one of two certifications: DSAT PADI Gas Blender Nitrox or DSAT PADI Trimix Blender.

Nitrox Made Easy: Which Nitrox system should I buy?
When deciding which Nitrox System to purchase there are a couple things you might take into consideration to help you make the best decision:
- Initial system procurement cost.
- Recurring cost per unit of Nitrox including maintenance, electricity and oxygen.
- Ease of system operation – effort and manpower required to operate.
- Availability to understand the types of costs associated with each of the different methods to make Nitrox.
You can use this spreHere is an additional spreadsheet to help you understand what it costs to fill a SCUBA tank at your shop. Tank Fill Cost Spreadsheet.
Joey Ridge Diver Services has an inexpensive downloadable Gas Blender Manual that has a wealth of information about Nitrox.
There are three major ways out there to make Nitrox. They are:
- Partial Pressure Blending (Enriched Air Nitrox – EANx).
- Nitrogen Separation Membrane (De Nitrogenated Air- DNAx).
- Continuous Gas Blending with the Nitrox Stik (also EANx).
There are advantages and disadvantages to each of the systems, here’s a quick run-down of their strengths and weaknesses:
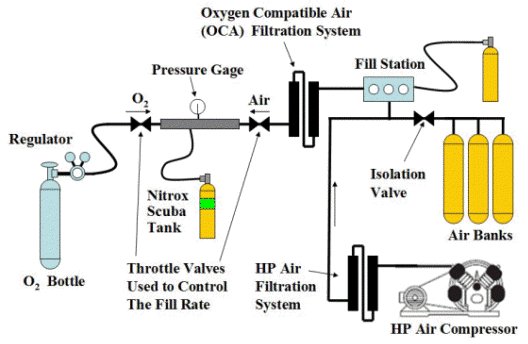
Nitrox Partial Pressure Blending:
Mixes pure oxygen directly into an empty and oxygen clean certified SCUBA tank, then topped off with with Oxygen Compatible Air (OCA) from banks to create desired oxygen mixture. Depending on the complexity of the blending station, you will spend a couple thousand dollars on the mixing panel and additional filtration system. Bottled oxygen needs to be obtained from your local gas Gas Distributor.

Nitrox Panel Membrane System – Coltri Sub
- Simple complete “Turnkey” operation.
- Adjust the regulated input to supply the desired oxygen %.
- Only 1-hour operator training required.
- 2 hour average installation.
- No compressor modifications.
- Reliable mix accurate to one tenth of 1%.
- Up to 20 year life.
- Produces from 21% to 40% oxygen.
- Unlimited continuous on-site production.
- Low pressure or high pressure supply.
- No size or flow limits.
- Banked, hot fills, or continuous nitrox output.
- Ideal for remote sites and liveaboards.
- Compact and lightweight.
- Trimix systems available.
- Oxygen levels of up to 40%.
- High volume output means no waiting for fills.
- Easy maintenance (1 inexpensive filter change each quarter).
- Operates on Grade D or E Air.
- Use your existing oil lubricated compressor (some compressors may not be suitable for nitrox use).
This Nitrox System uses semi permeable membranes to produce oxygen rich air (nitrox). The nitrox, at up to 40% oxygen, can then be compressed with an oil lubricated high-pressure compressor into scuba bottles or storage tanks for later use or with a low-pressure compressor for immediate delivery to divers.
The “New & Improved” membrane system is up to 20% more efficient and easier to use. The system requires an air source to supply air to the membrane for separation. This air source (supply air) can be from high-pressure storage tanks or from a low-pressure compressor.
First, the supply air pressure must be reduced to 80-300 PSI for use in the membrane. A regulator is used to adjust the input pressure and volume of nitrox to be made. After the regulator the air travels through filtration to ensure a proper air quality that will not damage or plug the membrane fibres. After the filtration the air is heated to a stable temperature that is constant and optimal for the membrane permeation. This temperature is about 110 degrees F. After the heater the air enters the membrane.
The membrane is made up of thousands of hollow fibres. Oxygen permeates faster than nitrogen through these fibres. At the nitrogen outlet there is a fixed orifice that will allow the right amount of nitrogen to escape at maximum output to produce about 43% oxygen at the permeate outlet (oxygen rich gas). The permeate exits the membrane into a static mixing tube that allows ambient air to mix with permeate. The gas mixture is then analysed with an in-line sensor before delivery to the compressor for compression.
As the operator increases the input pressure on the regulator, the volume of nitrox produced is increased as well as the oxygen % of the total gas mix. Decreasing input pressure will lower the nitrox oxygen % mix.
This system requires a separate LOW PRESSURE COMPRESSOR or a LOW PRESSURE STORAGE TANK to operate the membrane
